FDA Approves First Artificial Iris Implant
The U.S. Food and Drug Administration (FDA) has approved the implantation of the first artificial iris, developed by the German company HumanOptics AG. This announcement was published on the FDA’s official website.
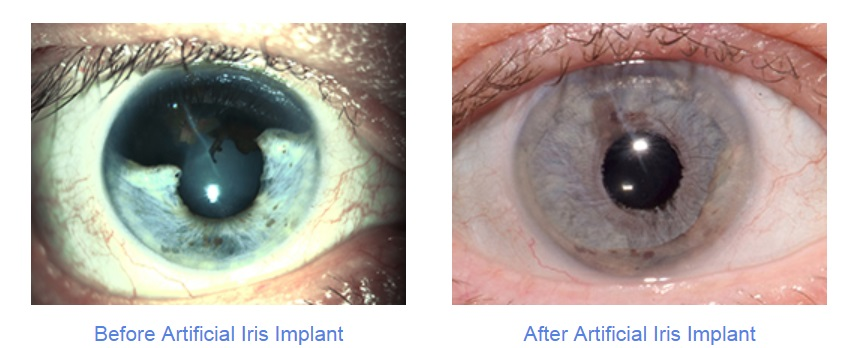
About the CustomFlex Artificial Iris
The CustomFlex Artificial Iris is made from thin, flexible medical-grade silicone. Each implant is custom-sized to fit the individual structure of the patient’s eye, and the color can be chosen according to the patient’s preference. To implant the artificial iris, the surgeon only needs to make a single 2.75-millimeter incision in the eye’s membrane. In most cases, the implant stays in place without the need for stitches.
Who Can Benefit from the Implant?
This procedure is intended to help people whose iris has been damaged or surgically removed, for example, due to melanoma. It is also recommended for patients with various forms of albinism, where the iris lacks pigment, and for those with aniridia—a rare condition in which the iris is partially or completely absent. Since the iris controls the amount of light entering the eye, people with aniridia often suffer from light sensitivity, cataracts, glaucoma, and other serious eye conditions.
Clinical Trials and Results
The artificial iris was tested on approximately 400 volunteers. After the transplant, 70% reported reduced light sensitivity and improved quality of life, while 94% were satisfied with the appearance of the implant.
Related Developments
Recently, scientists from Newcastle University in the UK announced that they had 3D-printed an artificial cornea, which could potentially be used for transplants in the future.